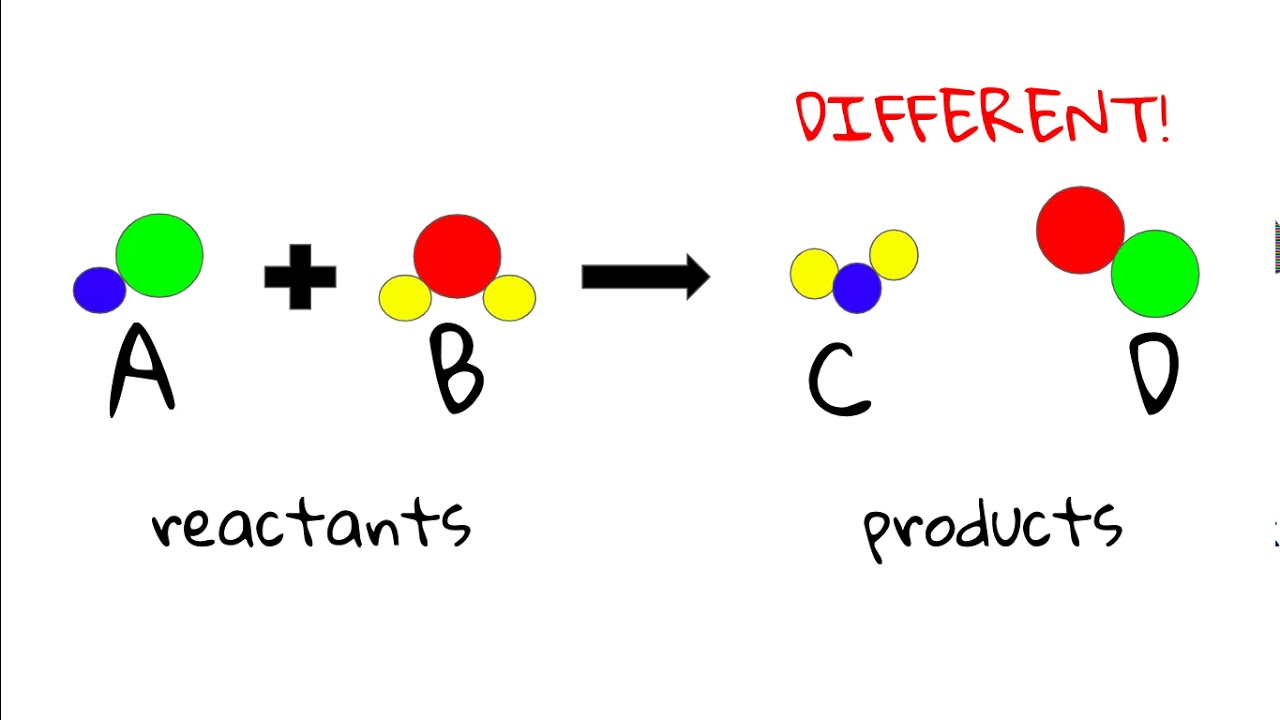
What are Reactant and Product?
A reactant is a substance that takes part in a chemical reaction and is consumed or transformed during the process. Reactants are found on the left side of a chemical equation and undergo chemical changes to form new substances. Conversely, a product is the substance that is formed as a result of the chemical reaction. Products appear on the right side of the chemical equation and are the end result of the interaction between reactants. The chemical changes lead to the creation of products with different properties from the original reactants.
When you use a natural gas stove, methane (CH4) from the gas supply reacts with oxygen (O2) from the air. In this reaction, methane and oxygen are the reactants that combine to produce carbon dioxide (CO2) and water (H2O). In the reaction between vinegar and baking soda, the reactants are vinegar and baking soda, which produce carbon dioxide gas, water, and sodium acetate as products. The fizzing and bubbling you see are caused by the carbon dioxide gas, which is one of the products of the reaction.
Why is it essential to learn Reactant and Product ?
Knowing about reactants and products is crucial for safely handling chemicals. For instance, in a household cleaning product, understanding the reactants (such as bleach) and the products (like chlorine gas) helps ensure safe use and storage. If bleach is mixed with ammonia, toxic chloramine vapors are produced. Being aware of the products formed in chemical reactions helps prevent dangerous situations and promotes safer practices in both domestic and industrial settings.
n industries, optimizing chemical reactions can improve efficiency and reduce costs. For example, in the manufacturing of fertilizers, understanding the reactants (like nitrogen and hydrogen) and the products (ammonia) allows chemists to adjust conditions to maximize yield and minimize waste. This knowledge helps in scaling up reactions from the laboratory to large-scale production while maintaining cost-effectiveness and environmental responsibility.
Knowledge of reactants and products is vital for assessing the environmental and health impacts of chemical processes. For instance, when burning fossil fuels, the reactants are hydrocarbons and oxygen, and the products include carbon dioxide and other pollutants. Understanding these reactions helps in developing strategies to mitigate environmental pollution and address health concerns related to air quality. By analyzing the products of chemical reactions, scientists and policymakers can work towards reducing harmful emissions and promoting sustainable practices.
Types of Reactant
Single Elements and Simple Compounds
Single elements and simple compounds often act as reactants in chemical reactions. Elements such as hydrogen (H₂), oxygen (O₂), and carbon (C) can react with other elements or compounds to form more complex substances. Simple compounds, like sodium chloride (NaCl) or sulfur dioxide (SO₂), can also be reactants. For example, in the reaction between hydrogen and oxygen to form water, both hydrogen and oxygen are single-element reactants:
2H2 + O2 -> 2H2O
Here, hydrogen gas and oxygen gas are the reactants that combine to produce water.
Acids and Bases
Acids and bases are another type of reactant commonly involved in chemical reactions, especially in neutralization reactions. Acids, like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), react with bases such as sodium hydroxide (NaOH) to form salts and water. For instance, the reaction between hydrochloric acid and sodium hydroxide produces sodium chloride (table salt) and water:
HCl + NaOH -> NaCl + H2O
In this reaction, HCl and NaOH are the reactants that neutralize each other to form salt and water.
Organic Compounds
Organic compounds, which contain carbon and hydrogen (and often oxygen, nitrogen, or other elements), are crucial reactants in many chemical reactions, particularly in organic chemistry. Examples include hydrocarbons like methane (CH₄), ethanol (C₂H₅OH), and glucose (C₆H₁₂O₆). In combustion reactions, organic compounds react with oxygen to produce carbon dioxide and water. For instance, the combustion of methane is represented by:
CH4 + 2O2 -> CO2 + 2H2O
Here, methane and oxygen are the reactants that produce carbon dioxide and water.
Redox Reactants
Redox (reduction-oxidation) reactions involve reactants that undergo changes in oxidation states. These reactions are characterized by the transfer of electrons between reactants. For example, in the reaction between zinc and copper sulfate:
Zn + CuSO4 -> ZnSO4 + Cu
Zinc (Zn) and copper sulfate (CuSO4) are the reactants. Zinc is oxidized (loses electrons) and copper (II) ions are reduced (gain electrons), resulting in the formation of zinc sulfate (ZnSO4) and copper metal.
Catalysts
Although not consumed in the reaction, catalysts can also be considered as reactants in a broader sense because they facilitate the chemical reaction without being changed themselves. For example, enzymes in biological systems act as catalysts to speed up biochemical reactions, such as the breakdown of substrates into products. In the industrial process of hydrogenation, catalysts like platinum or nickel are used to convert unsaturated fats into saturated fats:
Unsaturated Fat + H2 -> Saturated Fat
Here, the catalyst helps drive the reaction but is not consumed.
Research and Studies
The Discovery of the Law of Conservation of Mass by Antoine Lavoisier (1789)
Antoine Lavoisier, often called the "Father of Modern Chemistry," made one of the most fundamental contributions to the study of chemical reactions with his discovery of the Law of Conservation of Mass. In 1789, Lavoisier demonstrated that during a chemical reaction, the total mass of the reactants equals the total mass of the products. This principle established that mass is neither created nor destroyed in a chemical reaction. Lavoisier's work laid the foundation for modern chemistry, allowing scientists to predict the outcomes of chemical reactions with greater accuracy and leading to the development of stoichiometry, which is essential for balancing chemical equations and understanding reaction mechanisms. More about Law of Conservation of Mass
Development of Mass Spectrometry (1918)
John Dalton's atomic theory, published in 1808, revolutionized the understanding of chemical reactions by introducing the concept that matter is composed of indivisible atoms. Dalton proposed that atoms of different elements combine in fixed ratios to form compounds and that chemical reactions involve the rearrangement of these atoms. This theory provided a scientific explanation for the observations made by Lavoisier and others, offering a molecular-level understanding of chemical reactions. Dalton's atomic theory is a cornerstone of modern chemistry and has been crucial in advancing the study of chemical reactions, including the development of the periodic table and molecular chemistry. More about Atomic Theory by John Dalton
Jacob Berzelius’s Contribution to Chemical Symbols (1813))
Jacob Berzelius introduced the modern system of chemical notation and symbols, which greatly facilitated the study of chemical reactions. By using symbols to represent elements and compounds, Berzelius enabled scientists to write and balance chemical equations more systematically. His notation system made it easier to track reactants and products in chemical reactions, promoting clarity and precision in chemical research. More about Catalysis
The Discovery of Catalysis by Wilhelm K. Roelands (1899)
Wilhelm K. Roelands made significant contributions to the understanding of catalysis, the process by which the rate of a chemical reaction is increased by a substance called a catalyst. His research elucidated how catalysts interact with reactants to speed up reactions without being consumed. This discovery has had profound implications for industrial chemistry and has led to the development of various catalytic processes used in manufacturing and environmental applications. More about Catalysis
Facts about Chemical Reactants
Catalysts are substances that increase the rate of a chemical reaction by lowering the activation energy required. A lesser-known fact is that catalysts themselves are not consumed or permanently altered during the reaction. For example, in the catalytic conversion of hydrogen and oxygen to form water, catalysts like platinum or palladium speed up the reaction without being used up, highlighting their role in facilitating reactions without changing the overall stoichiometry.
Many chemical reactions are reversible, meaning they can proceed in both forward and backward directions. A lesser-known fact is that these reactions often reach a state of dynamic equilibrium, where the rate of the forward reaction equals the rate of the reverse reaction. This dynamic balance means that the concentrations of reactants and products remain constant over time, a concept crucial for understanding systems like enzyme catalysis and chemical synthesis.
The concentration of reactants can significantly affect the rate of a chemical reaction, a principle known as the rate law. A lesser-known fact is that the effect of concentration varies depending on the reaction order. For example, in a second-order reaction, doubling the concentration of one reactant will quadruple the reaction rate, which is critical for accurately predicting and controlling reaction rates in industrial and laboratory settings.
The solvent in which reactants are dissolved can greatly influence their reactivity. A lesser-known fact is that the solvent can affect the rate and outcome of reactions by stabilizing or destabilizing the reactants and transition states. For instance, in reactions involving ionic compounds, solvents like water can facilitate the dissociation of reactants into ions, thus enhancing their reactivity through ionic interactions.
Isomerization is a process where a reactant molecule is transformed into another molecule with the same chemical formula but a different structure (isomer). A lesser-known fact is that isomerization reactions are often crucial in industrial processes and natural biochemical pathways. For example, in the production of biofuels, the isomerization of alkanes can improve the fuel’s performance by altering its physical properties.
Quiz
1. How did the Hubble Deep Field observation in 1995 change our understanding of the universe?
2. Describe one contribution of ancient Babylonians to early astronomy.
3. Explain the impact of the Islamic Golden Age on the development of astronomy during the medieval period.
4. How did the heliocentric model proposed by Copernicus revolutionize our understanding of the solar system?
5. What are some of the key research areas in contemporary astronomy, and why are they significant?